

![]()
MCT HbsAg/HCV/HIV 3 Multi Combo Test is an in vitro, rapid, self performing, qualitative two site sandwich immunoassay used for the detection of antibodies to HCV , HIV 1 / 2 virus and HBsAg in human serum / plasma or whole blood specimens.


![]()
MCT HbsAg/HCV/HIV 3 Multi Combo Test is an in vitro, rapid, self performing, qualitative two site sandwich immunoassay used for the detection of antibodies to HCV , HIV 1 / 2 virus and HBsAg in human serum / plasma or whole blood specimens.
MCT HbsAg/HCV/HIV 3 Multi Combo Test is using an immunochromatography method for the detection of antibodies to HCV , HIV 1 / 2 virus and HBsAg in human serum/plasma and whole blood. Hepatitis C virus (HCV) is a small, enveloped, and single-stranded RNA virus. It is the major cause of parenterally transmitted non-A, non-B hepatitis. Antibodies to HCV are reported in 80% of the non-A, non-B hepatitis patients. Blood containing the Hepatitis B Virus (HBV) is potentially infectious. Hepatitis B Surface Antigen (HBsAg), earlier known as Australia Antigen, is among the first serological markers that circulate in the blood of infected persons even two to three weeks prior to the appearance of clinical symptoms. The levels of HBsAg are especially elevated during the symptomatic phase and decline thereafter. Detection of HBV using HBsAg as the marker to screen blood donors is essential to reduce the risk of transmission of Hepatitis B by blood transfusion. HBsAg detection is also useful for screening high risk groups for HBV and for differential diagnosis of Hepatitis infections. Core HBsAg detects the presence of HBsAg in serum/plasma specimens, qualitatively, at concentrations as low as 0.5 ng/ml within 15 minutes. Highly purified antigen of gp 41, representing HIV- 1 and gp 36 representing HIV-2, recombinant HCV antigens (Core, NS-3, NS-4 and NS-5) and Anti HBsAg antibodies are used in this test.
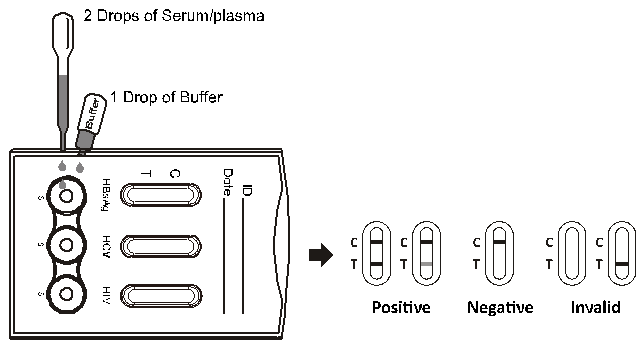
MCT HbsAg/HCV/HIV 3 Multi Combo Test utilizes the principle of immunochromatography, a unique two site immunoassay on a membrane. In MCT HbsAg/HCV/HIV 3 Multi Combo Test, a line containing a mixture Highly purified antigen of gp 41, representing HIV- 1 and gp 36 representing HIV-2 ( line 1) , a line of Anti HBsAg antibodies (line2) and a line of recombinant HCV antigens (line 3) are coated on the membrane in the test region and anti- Rabbit antiserum at the control region. As the test sample flows through the membrane assembly within the test device, the colored conjugated colloidal gold complex (HIV 1 / 2 specific recombinant antigen-colloidal gold conjugate, anti-HBsAg-colloidal gold conjugate, HCV specific recombinant antigen-colloidal gold conjugate) reacts with antibodies to HIV 1 / 2, HCV and HBsAg in the sample. This complex moves further on the membrane to the test region where it is immobilized at individual lines coated with the HIV 1 / 2 Specific recombinant antigens ( line 1) , Anti HBsAg antibodies (line2) and recombinant HCV antigens (line 3) coated on the membrane leading to formation of a colored band which confirms a positive test result. Absence of this colored band in the test region indicates a negative test result. The unreacted conjugate and unbound complex if any move further on the membrane and are subsequently immobilized by the anti-rabbit antibodies coated on the membrane at the control region, forming a colored band. This control band serves to validate the test results.
The products prices is normally including the following parts:
As the prices may varied from time to time and region to region, please contact us to get the full details. [email protected]
The different terms in contract is simply as below, which is defined in Incoterms 2010:
For more details you can find it on the Wiki page. https://en.wikipedia.org/wiki/Incoterms
Hangzhou MED Technology Co., Ltd.
Normally the lead time would be around 4 to 6 weeks after deposit received. And the freight time would depend on the method. Finally plus the local customs time cost.
Hangzhou MED Technology Co., Ltd.
The lab reference tests result is different from test to test. Please contact us to get more information about it. You will have the response as soon as possible. [email protected]
Hangzhou MED Technology Co., Ltd.
The shelf life is from 12 to 36 months.
Hangzhou MED Technology Co., Ltd.
The guarantee is as long as the shelf life. And base on our traceability system, all products can be tracked back to the original materials.
Hangzhou MED Technology Co., Ltd.
